A digital twin of the aorta to prevent aneurysm rupture
15,000 Europeans die each year from rupture of an aneurysm in the aorta. Stéphane Avril and his team at Mines Saint-Étienne are working to better prevent this. To do so, they develop a digital twin of the artery of a patient with an aneurysm. This 3D model makes it possible to simulate the evolution of an aneurysm over time, and better predict the effect of a surgically-implanted prosthesis. Stéphane Avril talks to us about this biomechanics research project and reviews the causes for this pathology along with the current state of knowledge on aneurysms.
Your research focuses on the pathologies of the aorta and aneurysm rupture in particular. Could you explain how this occurs?

Stéphane Avril
Stéphane Avril: The aorta is the largest artery in our body. It leaves the heart and distributes blood to the arms and brain, goes back down to supply blood to the intestines and then divides in two to supply blood to the legs. The wall of the aorta is a little bit like our skin. It is composed of practically the same proteins and the tissues are very similar. It therefore becomes looser as we age. This phenomenon may be accelerated by other factors such as tobacco or alcohol. It is an irreversible process that results in an enlarged diameter of the artery. When there is significant dilation, it is called an aneurysm. This is the most common pathology of the aorta. The aneurysm can rupture, which is often lethal for the individual. In Europe, some 15,000 people die each year from a ruptured aneurysm.
Can the appearance of an aneurysm be predicted?
SA: No, it’s very difficult to predict where and when an aneurysm will appear. Certain factors are morphological. For example, some aneurysms result from the malformation of an aortic valve: 1 % of the population has only two of the three leaflets that make up this part of the heart. As a result, the blood is pumped irregularly, which leads to a microinjury on the wall of the aorta, making it more prone to damage. One out of two individuals with this malformation develops an aneurysm, usually between the ages of 40 and 60. There are also genetic factors that lead to aneurysms earlier in life, between the ages 20 and 40. Then there are the effects of ageing, which make populations over 60 more likely to develop this pathology. It is complicated to determine which factors predominate in relation to one another. Especially since if at 30 or 40 an individual is declared healthy and then starts smoking, which will affect the evolution of the aorta.
If aneurysms cannot be predicted, can they be treated?
SA: In biology, extensive basic research has been conducted on the aortic system. This has allowed us to understand a lot about what causes aneurysms and how they evolve. Although specialists cannot predict an aneurysm’s appearance, they can say why the pathology appeared in a certain location instead of another, for example. For patients who already have an aneurysm, this also means that we know how to identify the risks related to the evolution of the pathology. However, no medication exists yet. Current solutions rely rather on surgery to implant a prosthesis or an endoprosthesis — a stent covered with fabric — to limit pressure on the damaged wall of the artery. Our work carried out with the Sainbiose joint research unit [run by INSERM, Mines Saint-Étienne and Université Jean Monnet], focused on gathering everything that is known so far about the aorta and aneurysms in order to propose digital models.
What is the purpose of these digital models?
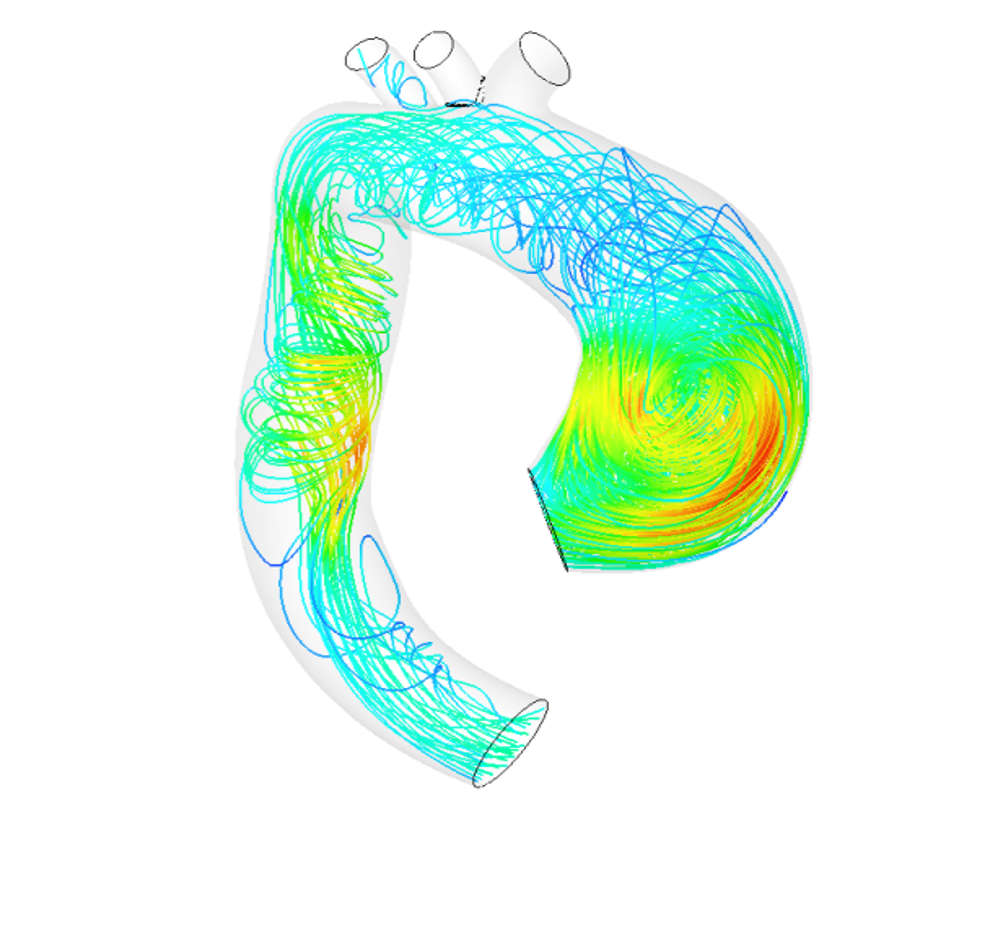
SA: The model should be seen as a 3D digital twin of the patient’s aorta. We can perform calculations on it. For example, we study how the artery evolves naturally, whether or not there is a high risk of aneurysm rupture, and if so, where exactly in the aorta. The model can also be used to analyze the effect of a prosthesis on the aneurysm. We can determine whether or not surgery will really be effective and help the surgeon choose the best type of prosthesis. This use of the model to assist with surgery led to the creation of a startup, Predisurge, in May 2017. Practitioners are already using it to predict the effect of an operation and calculate the risks.
Read on IMTech: Biomechanics serving healthcare
How do you go about building this twin of the aorta?
SA: The first data we use comes from imaging. Patients undergo CAT scans and MRIs. The MRIs give us information about blood flow because we can have 10 to 20 photos of the same area over the duration of a cardiac cycle. This provides us with information about how the aorta compresses and expands with each heart beat. Based on this dynamic, our algorithms can trace the geometry of the aorta. By combining this data with pressure measurements, we can deduce the parameters that control the mechanical behavior of the wall, especially elasticity. We then relate this to the composition of elastin, collagen and the smooth muscle cell ratio of the wall. This gives us a very precise idea about all the parts of the patient’s aorta and its behavior.
Are the digital twins intended for all patients?
SA: That’s one of the biggest challenges. We would like to have a digital twin for each patient as this would allow us to provide personalized medicine on a large scale. This is not yet the case today. For now, we are working with groups of volunteer patients who are monitored every year as part of a clinical study run by the Saint-Étienne University hospital. Our digital models are combined with analyses by doctors, allowing us to validate these models and talk to professionals about what they would like to be able to find using the digital twin of the aorta. We know that as of today, not all patients can benefit from this tool. Analyzing the data collected, building the 3D model, setting the right biological properties for each patient… all this is too time-consuming for wide-scale implementation. At the same time, what we are trying to do is identify the groups of patients who would most benefit from this twin. Is it patients who have aneurysms caused by genetic factors? For which age groups can we have the greatest impact? We also want to move towards automation to make the tool available to more patients.
How can the digital twin tool be used on a large scale?
SA: The idea would be to include many more patients in our validation phase to collect more data. With a large volume of data, it is easier to move towards artificial intelligence to automate processing. To do so, we have to monitor large cohorts of patients in our studies. This means we would have to shift to a platform incorporating doctors, surgeons and researchers, along with imaging device manufacturers, since this is where the data comes from. This would help create a dialogue between all the various stakeholders and show professionals how modeling the aorta can have a real impact. We already have partnerships with other IMT network schools: Télécom SudParis and Télécom Physique Strasbourg. We are working together to improve the state of the art in image processing techniques. We are now trying to include imaging professionals. In order to scale up the tool, we must also expand the scope of the project. We are striving to do just that.
Around this topic on I’MTech